- Lewis Dot Structure Calculator Online Scientific Calculator
- Lewis Dot Structure Calculator Online Desmos
- Lewis Dot Structure Calculator Online Tool
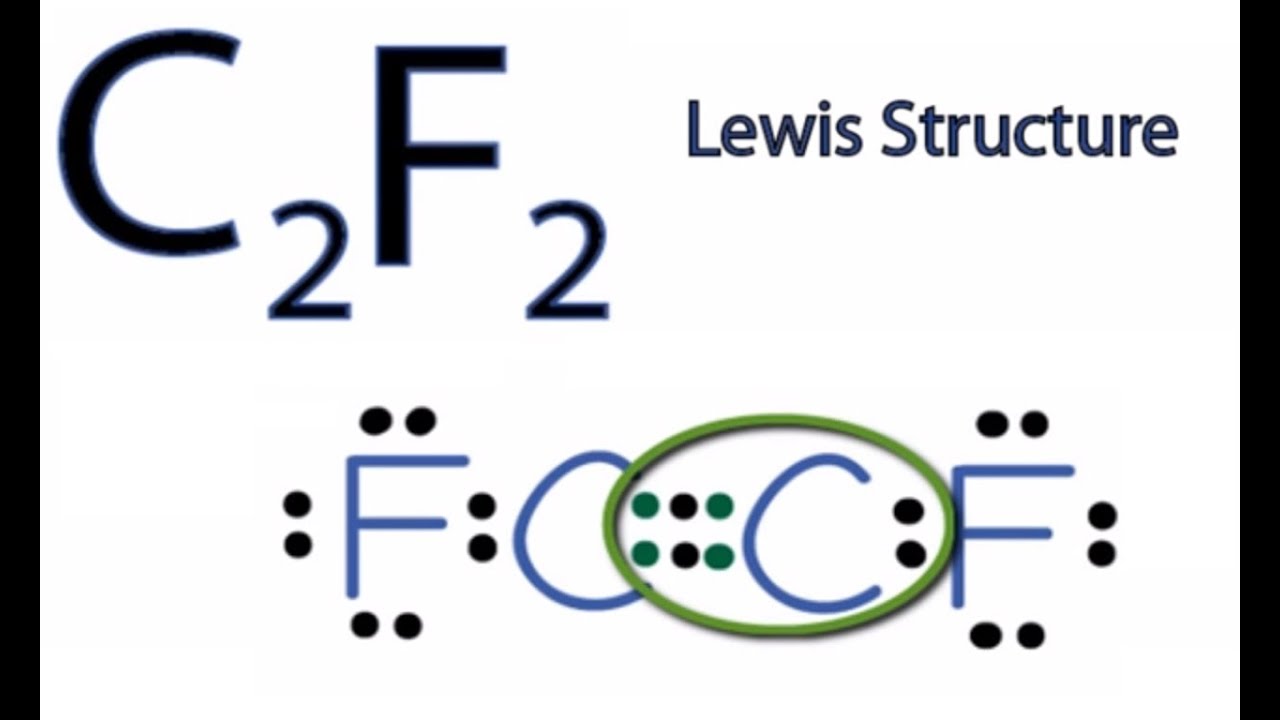
When drawing Lewis structure to be valid, each atom must have a full octet of 8 electrons (except hydrogen, which only has a duet of 2 electrons). The structure with both of the hydrogens and both of the fluorines bonded to the carbon allows all atoms to have the proper number of electrons (if the lone pairs on the fluorine atoms are included). In the structure below, fluorine has six electrons in three lone pairs (not drawn) and the seventh electron participating in a covalent single bond to the Carbon, which allows the Fluorine to have an octet. Likewise, the carbon achieves its octet by sharing bonds with the two F and two H allows. Hope this helps.
Generating the Lewis dot Structure. To generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. In this step, add the total count of valence electrons from all the atoms in a bit. Find the required count of. Lewis Dot Structure. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as “electron bookkeeping”. In Lewis dot structures each dot represents an electron. A pair of dots between. Drawing Lewis Structures - Oneonta.
Get the free 'Lewis Structure Finder' widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram Alpha.
Lewis Dot Structure Calculator Online Scientific Calculator
F
I
H-C-H
Lewis Dot Structure Calculator Online Desmos
I
F
Lewis Dot Structure Calculator Online Tool
|